The Translational Laboratory serves both the preclinical investigator and the clinical researcher. Preclinically, the Translational Lab has the capacity to undertake in vitro based assays, in vivo evaluation of compound activity in tumor models including athymic, severe combined immunodeficient, and immunocompetent models and pharmacodynamic assays for in vivo assays. We are versed in all aspects of molecular target evaluation, including cell cycle analysis, gene expression at the RNA and protein level (realtime PCR analyses, Western blotting, immunohistochemistry), and collaboration with the Biopolymers shared resource of the UMGCC for genomic studies.
To support the clinicians, the Translational Laboratory develops pharmacodynamic markers of drug effect suitable for a collaborating and clinical routine laboratory to process subsequent clinical specimens. Additionally, the Translational Laboratory will receive clinically derived specimens and process them (e.g., protein, RNA and DNA isolation) for use within the lab or to distribute to collaborators. One major output of the our work is data for interpretation of clinical trials results, e.g. correlation of an effect of a drug on patient surrogate or tumor cells with achievable pharmacology in early phase trials. A second major output is preliminary pre-clinical data that would support the development of a full fledged clinical trials opportunity.
Services Provided
- In Vitro Based Assays
- In Vivo Mouse Assays
- Analyses of pharmacodynamic (PD) endpoints in patient samples, tumor or surrogate tissues as well as preclinical samples
- Processing of Clinical Trial Material
In Vitro Based Assays
The Translational Laboratory provides a spectrum of established in vitro proliferation assays that are all automated and run in a multi-well format to enable high-throughput testing of potentially novel and established anticancer agents in cell-based screens. Over 40+ permanent tumor and normal cell lines are available, which have been characterized for a large number of currently highly attractive molecular targets.
Proliferation assays that can be performed with these cell lines include :
- WST-1 assay (MTT_like, methyl tetrazolium assay, detecting mitochondrial function),
- Alamar Blue (measures metabolic activity by redox reactions) – both colorimetric and fluorometric
- SRB assay (measuring total cellular protein, assay used by the NCI 60 cell line screen),
- propidium iodide (determining total DNA content).
- Viability (trypan blue exclusion)
WST1 Cell Proliferation Assay
 IC50 generation: MDA-MB-231 human breast cancer cells were plated in a 96-well plate. 24 hrs later, drug was added (dose response). 72 hrs later, assay was terminated with WST-1 (Roche) and read on Bio-Tek Synergy HT plate reader. The data was plotted on GraphPad Prism and the software generates an IC50. Figure: Generation of IC50 values
IC50 generation: MDA-MB-231 human breast cancer cells were plated in a 96-well plate. 24 hrs later, drug was added (dose response). 72 hrs later, assay was terminated with WST-1 (Roche) and read on Bio-Tek Synergy HT plate reader. The data was plotted on GraphPad Prism and the software generates an IC50. Figure: Generation of IC50 values
Other Cell-Based Assays
xCELLigence Real-time System: measures cell proliferation, migration and invasion in real time. Useful for drug inhibition of proliferation, migration and invasion and assessment of these endpoints of knock-down or over-expression of gene of interest
| VIBO conc | Unit | Color |
|---|---|---|
| 0.00E+00 | ppq | red |
| 0.00E+00 | pM | lt green |
| 50 | nM | dk blue |
| 500 | nM | pink |
| 1 | uM | br blue |
| 2.5 | uM | purple |
| 5 | uM | orange |
| 10 | uM | dk green |
xCELLigence Migration Assay
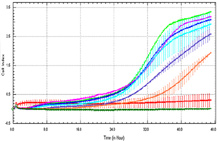 xCELLigence migration assay: Inhibition of migration of HCT 116 cells with increasing concentration of Drug X. Cell Index vs time. Decreased cell index indicative of less cells migrating through membrane.
xCELLigence migration assay: Inhibition of migration of HCT 116 cells with increasing concentration of Drug X. Cell Index vs time. Decreased cell index indicative of less cells migrating through membrane.
Potentiation Studies
Potentiation studies tests the effect of one drug on another. A fixed concentration (e.g., IC30) of one compound is added to a dose response of a second compound. Compound can be added concurrently or sequentially with WST-1, alamar blue or SRB as endpoint.
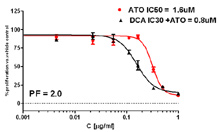 Potentiation Assay: The effect of IC30 Dichloroacetate (DCA) on a dose response of Arsenic Trioxide (AT0). DCA was added 24 hours prior to ATO. The Potentiation Factor (PF) of DCA on the IC50 of ATO was 2.0.
Potentiation Assay: The effect of IC30 Dichloroacetate (DCA) on a dose response of Arsenic Trioxide (AT0). DCA was added 24 hours prior to ATO. The Potentiation Factor (PF) of DCA on the IC50 of ATO was 2.0.
Emadi et al AACR Proceedings 2013
Synergy Studies
The MTT assay is further employed for drug combination studies that are performed based on the fixed IC50 ratio method by Chou and Talalay. For processing the data resulting from combination studies, we have the CalcuSyn software package available that enables us to determine combination indices and other relevant parameters with ease.
| ED50 | ED75 | ED900 | |
| Trial 1 | 0.818 | 0.594 | 0.369 |
| Trial 2 | 0.789 | 0.562 | 0.4 |
| CI Avg | 0.803±0.021 | 0.556±0.009 | 0.385±0.022 |
In this experiment leukemia cells were treated with fixed ratios of the IC50 value for two compounds, DCA and ATO, for 72 hours. After termination (WST-1 addition), the OD values were evaluated by the CalcuSyn software. The Combination Index, CI, for the ED50, ED75 and ED90 for this experiment show synergy. CI<1 are synergistic, CI=1 are additive, CI>1 are antagonistic. Published by Emadi et al AACR Proceedings 2013
Angiogenesis
In a 96 well format, HUVEC cells are plated on matrigel and allowed to differentiate to form ‘tubes’. Dose response of therapeutic is added to media. Each condition is repeated in triplicate.
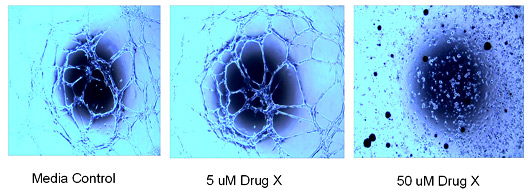
Angiogenesis Tube Formation Assay: Effect of drug on the tube formation of HUVEC cells on matrigel. Cells were plated and drug was added immediately – exposure for 24 hours.
In Vivo Mouse Assays
The Translational Laboratory has an IACUC approved umbrella animal use protocol "Animal Models for Studying Tumor Biology and for the Evaluation of Pharmacodynamic and Pharmacokinetic Endpoints of Novel and Experimental Cancer Therapies" approved for three years (#011-1008 till Jan 2014). Any tumor line or drug can be used in the following approved procedures considered key in preclinical anticancer drug development:
- Tolerability: Determination of the maximal tolerated dose (MTD) of a novel agent
- Tumor Growth: Establishment of human tumor xenografts or murine tumor lines from model cell lines (e.g. target transfected) and tissues
- Pharmacokinetics: Generation of plasma and single or multiple injection for pharmacokinetic studies
- Efficacy (flank models): Tumor efficacy experiments in traditional s.c. nude mouse xenografts of a test compound versus standard agents alone or in combination
- Efficacy (orthotopic models): similar to flank models except the tumors are in organ of cell origin (e.g., breast, prostate, other)
- Pharmacodynamic Endpoints: Determination of pharmacodynamic endpoints in xenograft efficacy models using serum or tumor tissue based parameters.
- Imaging of cells with Xenogen System – luciferase-tagged cells can be implanted orthotopically or injected IV
Because it is very complicated and difficult to obtain IACUC approval for investigators without prior and documented experience or experienced personnel in the field of animal research, the Translational Laboratory offers a fast and easy option to perform grant-related or contract-related in vivo experiments. New cell lines or drugs only require a notification of the animal care and use office through the submission of an amendment. The latter is mainly concerned with safety information and is assured fast track review and approval within days.
- Tolerability Studies tests the effect of a novel agent, or combination of agents on a whole organism. Mice or rats can be dosed intravenously, intraperitoneal, oral, subcutaneous. We suggest these studies should be dosed in the exact manner a future efficacy study would be dosed.
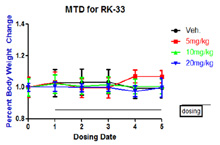
In Vivo Tolerability Assay: Mice were dosed IP for 5 days with three different concentrations of RK-33. No weight loss was observed dosing period. Mice were followed for one more week (data not shown)
- Establishment of Tumors in Mice - Growth of tumors in mice allows for the investigator to determine the effect of knocking down gene expression or over-expressing a protein of interest on tumor initiation and growth.
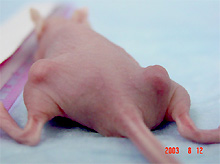
Tumor Formation: Mice are implanted with tumor cells in the presence of matrigel on right and/or left flanks. Tumor growth is monitored over time.
- Pharmacokinetics – tests the length of time a drug stays in the circulation of a mouse after systemic administration.
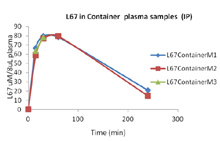
In Vivo Pharmacokinetic Analysis: Mice are dosed with a drug. At specific time points, mice (3) are euthanized and blood drawn and plasma isolated and snap frozen. Plasma is sent to a Bioanalytical lab for analysis. Graph on right is a novel drug tested at 15 min, 30 min, 1 hr and 4 hours post intraperitoneal injection. Three mice per time point were evaluated. Not published yet.
- Efficacy – flank models - Xenograft models allow investigators to test the effect of novel agents on established cell lines in vivo. Cells are injected subcutaneously on the flank of immunocompetent or immune-incompetent mice (nude, SCID, NSG). Drug treatment is started and tumor volume is monitored over time. Novel compounds can also be dosed in combination with known chemotherapeutics. Or, known chemotherapeutics can be used as a positive control.
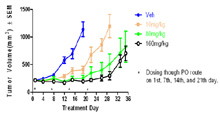
In Vivo Xenograft Efficacy: Effect of drug on human A375 melanoma tumor model in nude mice. Dosing started when tumors reached ~ 200mm3. Drug was dosed orally once per week for four weeks. Tumor volume was measured over time.
- Efficacy – orthotopic models - Injection of luciferase tagged cells either intravenously (IV) or via orthotopic injection (i.e., breast cells into mammary fat pad or prostate cells into prostate) allows the growth of cells to be followed over time using the Xenogen/IVIS system. Mice can be treated with compounds to inhibit growth of primary tumors or of metastases.
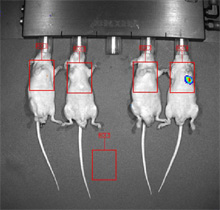
Human breast cancer cells were injected IV into the tail vein. Nine weeks post injection, one mouse of four has visible lung metastases.
Pharmacodynamic Endpoints. The effect of a compound on its intended target can be tested in vivo. This sort of experiment accomplishes two goals: (1) does the compound effect the expected protein target (e.g., enzyme inhibition) and (2) does the compound achieve the correct concentrations in its target tissue. The example below shows an increase in gamma-H2AX expression on a Western blot. A novel compound was dosed systemically and human breast cancer xenograft tissue was removed 4 hours post last dose.
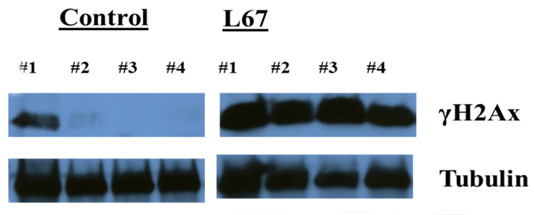
Mice were treated with L67 daily x3, tumors excised, tumors processed for protein lysates and then run on Western blot and probed for γH2Ax and Tubulin (not published yet)
- Imaging w/Xenogen Cells tagged with luciferase or GFP can be visualized using the Xenogen/IVIS system (on 6th floor HSFI). Cells can be injected orthotopically (site of origin) or IV and can then be visualized either short term (hours/days) or long term (weeks). Please see #5 above for an example.
PHARMACODYNAMIC ENDPOINTS – Clinical Trials
Analyses of pharmacodynamic (PD) endpoints in tumor or surrogate tissues and cell lines
Sample collection and processing for PD analyses is a core business of the Translational Laboratory. Services comprise the development of biochemical, molecular or cellular assays that are tailored and validated according the translational research question associated with a clinical trial, the development of standard operating procedures including instructions for research nurses and collection logistics. Assay technologies are first adapted to handling of micro quantities of patient materials by optimizing the procedures with human tumor xenograft and mouse tissues. They are mostly based on immunohistochemistry or immunofluorescence for tracking of target expression at various time points in cytospins or tissue microarrays. Western blotting for qualitative and quantitative evaluation of protein expression and real time PCR for mRNA expression are also frequently used.
Processing of Clinical Trial Material
The TLSS has experience processing the following tissues from human clinical trials:
- Plasma
- Serum
- Tumor Biopsy
- Whole Blood (isolation of PBMC, DNA, RNA, protein)
- Bone Marrow (isolation of marrow cells)
- Buccal Mucosa