Processing of Clinical Trial Material
The TLSR has experience processing the following tissues from human clinical trials:
- Plasma
- Serum
- Tumor Biopsy
- Whole Blood (isolation of PBMC, DNA, RNA, protein)
- Bone Marrow (isolation of marrow cells)
- Buccal Mucosa
Analyses of Pharmacodynamic (PD) Endpoints in Tumor or Surrogate Tissues
Sample collection and processing for PD analyses is a core business of the Translational Laboratory. Services comprise the development of biochemical, molecular or cellular assays that are tailored and validated according the translational research question associated with a clinical trial, the development of standard operating procedures including instructions for research nurses and collection logistics.
Assay technologies are first adapted to handling of micro quantities of patient materials by optimizing the procedures with human cell lines or tumor xenograft material. Immunohistochemistry or immunofluorescence for tracking of target expression at various time points in cytospins, western blotting for qualitative and quantitative evaluation of protein expression and real time PCR for mRNA expression are frequently used.
We also isolate primary leukemia cells and test the effect of compounds in culture. Endpoints include proliferation, viability, cell cycle (in collaboration with UMGCCC flow cytometry core), apoptosis (in collaboration with UMGCCC flow cytometry core), western blot and ELISA.
Example 1
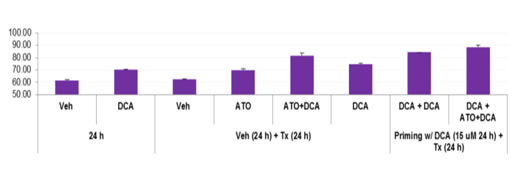
These were primary blast leukemia cells isolated from a patient. Ex vivo, cells were treated with arsenic trioxide and dichloroacetate to determine if the combination induces apoptosis as measured by Annexin V expression (flow cytometry)
From Emadi et al poster presented at ASH 2013; manuscript in preparation
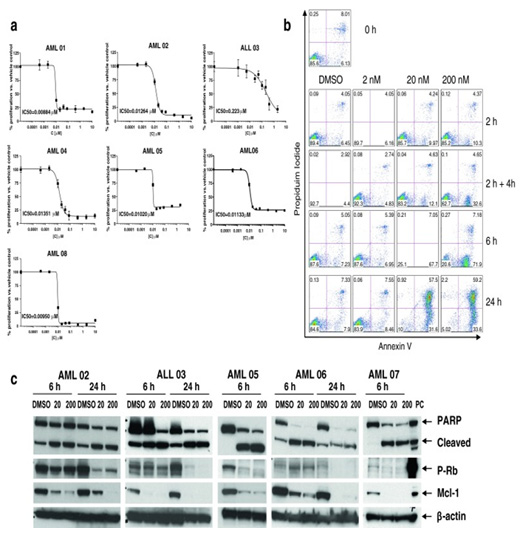
Cells isolated from leukemia patients. We determined IC50 of a novel CDK inhibitor (Merck) as well as effect on cell cycle and apoptosis induction as measure by PARP cleavage phosphorylation of Rb and decreased expression of mcl-1 by Western blot.
Figure 2 from Gojo et al Cancer Chemother Pharmacol 2013 72: 897-908.